POWDER
PROCESSES
Pressing And Sintering
Alternative Powder Processes
Process Design For Powder Metallurgy
MANUFACTURING
PROCESSES
Metal Casting
Metal Forming
Metal Rolling
Metal Forging
Metal Extrusion
Metal Drawing
Sheet Metal

|
Powder Metallurgy
Powder metallurgy is the manufacturing science of producing solid parts of desired geometry and material from powders. Commonly known as powder metallurgy, it may also be referred to as powder processing considering that non-metal powders can be involved. Powders are compacted into a certain geometry then heated, (sintered), to solidify the part. The manufacturing advantages and disadvantages, as well as the applications for parts produced by this method, are discussed latter in the design and applications of powder metallurgy section.
The first consideration in powder metallurgy is the powders used for the manufacturing process. Several different measures are used to quantify the properties of a certain powder. Powders can be pure elements or alloys. A powder might be a mixture of different kinds of powders. It could be a combination of elemental powders, alloy powders, or both elemental and alloy powders together. Material and the method of powder production are critical factors in determining the properties of a powder. It should always be remembered, when working with powders, that the powder itself may be a potential hazard. Some powders may be flammable and/or present health risks to workers. Safety precautions should always be taken when handling or storing powders. Also, be sure to follow any regulations regarding the handling, storage, or disposal of powders. Powder selection and processing will depend on cost, desired purity and mechanical properties of finished product. Environmental control is critical in proper storage and handling of powders. Contamination of powder can result in powder degradation and should be avoided. Remember, high surface areas cause powders to react readily with outside materials. This can have various results, oxidation for example, caused by oxygen present in the air.
Powder Properties
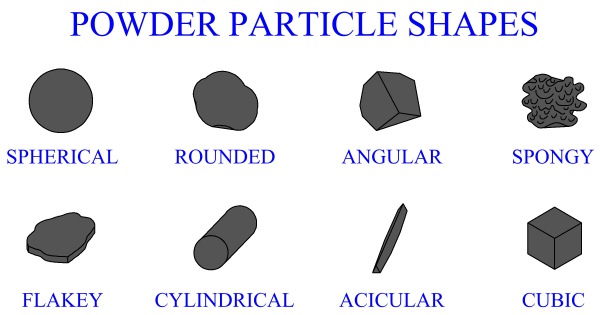
Powders are finely divided solid particulates. The size and shape of individual particulates is important. Characteristics of a powder can be quantified in several ways. These characteristics are necessary to understand when selecting a powder for an operation, since powder properties will affect processing factors.
Size And Distribution
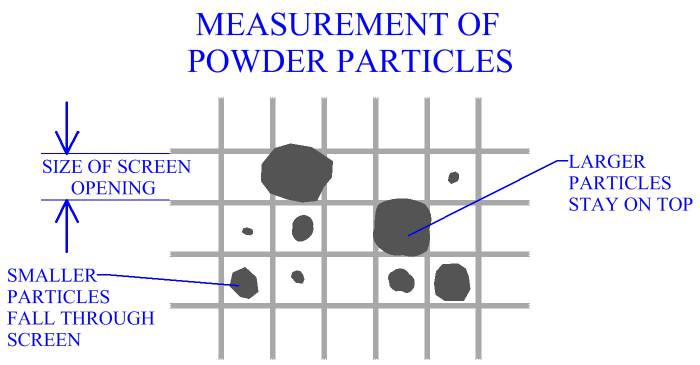
Size of particles is a factor that will affect processing of metal powders.
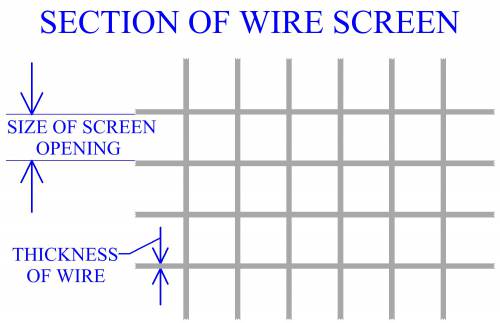
In manufacturing practice, powders are commonly measured using a series
of screens with different sized
openings. Each screen is a wire mesh with openings ideally of the same size.
Screens for powder measurement are designated according to the number of
openings per linear inch, (i.e. 30, 100). Openings per linear inch are the same
in the 2 dimensions of the screen's surface, therefore the number of openings
per square inch is the square of the linear number. A screen with a linear
measurement of 100 has 1002 or 10,000 openings per square
inch. When determining the size of an opening, the size of the screen's wire must
also be considered. Mesh opening size, (MS), can be determined by
MS = 1/MC - WS, where MC is the mesh count, (openings per
linear inch), and WS is the thickness of the wire.